

Last update: 14 July 2007

Custom Search
|
 Last update: 14 July 2007 |
|
 |
|
Left: an Early Devonian landscape;
right: two early tetrapods along the banks of a Late Devonian stream: Acanthostega gunneri (foreground) and Ichthyostega stensioei (in the background). |
||
|
|
|
|
|
|
|
|
|
|
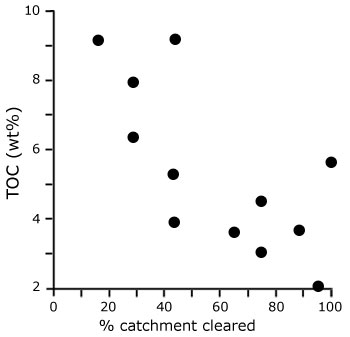
Furthermore, the rapidly expanding vegetation exerted a drag force in shallow water currents and decreased the
depositional energy of the colonised transitional habitats (not unlike the modern mangrove forests: Mazda et al., 1997a,b), thereby increasing the sedimentation rate of fine sediment. |
  |
|
|
 |
|
|
|
|
|
|
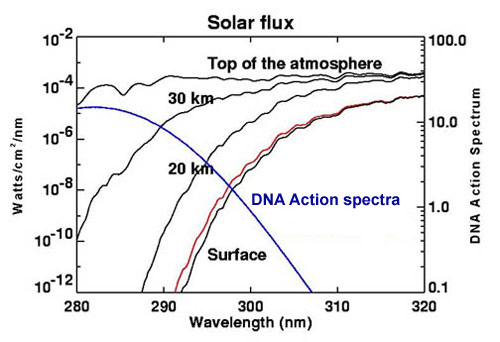 |
On the other hand, the increase in atmospheric oxygen resulted in a thickening of the
Devonian ozone layer, already formed by the photosynthetic action of unicellular autotrophs during the Proterozoic eon
(2500-540 millions of years ago: Bullini et al., 1998). |
|
|
How these dramatic changes affected the conditions of these shallow aquatic environments? That is, abundant trophic, environmental and metabolic resources, unexploited by vertebrate competitors, as well as habitats free from aquatic predators, were potentially available to any fish lineage that could increase its terrestriality (Clack, 2002). |
|
|
Evolution of the choanas in the tetrapodomorphs. |

All content on this website (including text, photographs, and any other original works), unless otherwise noted, is licensed under a
Creative Commons License