

Last update: 16 April 2010

Custom Search
|
 Last update: 16 April 2010 |
|
|
|
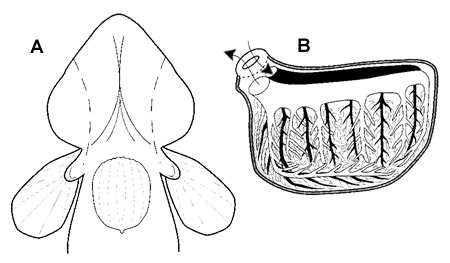 Air Breathing Organs (ABOs) of Pseudapocryptes lanceolatus. A: ventral view of head, pectoral and pelvic fins ; the dashed lines indicate the extension of expanded opercular pouches. B: medial view of the opercular circulatory system (arrows show the direction of blood flow). Modified from Graham, 1997, with permission from Elsevier. |
|
 |
|
|
|
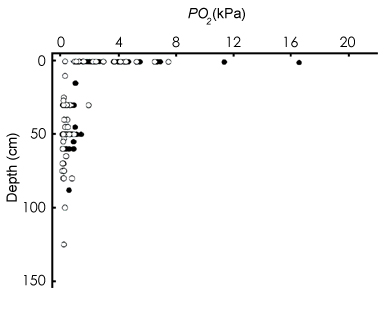
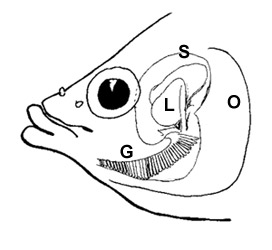
Several gobies can hold air bubbles
in the oral cavity while they are in water (AG= air gulping: Gee & Gee, 1995), but more terrestrial mudskippers (i.e. Periophthalmus
spp. and Periophthalmodon spp.) also do that whilst out of water, by sealing
opercular chambers through a ventro-medial valve (Sponder & Lauder, 1981;
Clayton, 1993; Martin & Bridges, 1999; Graham, 1997). These species mostly
rely on cutaneous respiration and on highly vascularised bucco-pharyngeal mucosae in air; wereas whilst they are in water, where O2 extraction is less efficient, they make use of
both branchial and cutaneous respiration (Clayton, 1993; Ishimatsu et
al., 1999; Takeda et al., 1999). |
|
|

All content on this website (including text, photographs, and any other original works), unless otherwise noted, is licensed under a
Creative Commons License